We are dedicated to restoring sight to the millions of people with corneal diseases through the advancement of therapies based on the inherent regenerative properties of naturally occurring corneal endothelial and epithelial cells.
One Molecule. Two Products. Multiple Potential Indications.
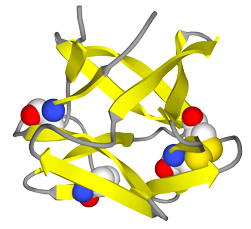
Trefoil is advancing two products based on TTHX1114 for people with a spectrum of corneal diseases affecting either the front (epithelial) or the back (endothelial) surface of the cornea. The company’s lead candidate, TTHX1114, is an engineered form of Fibroblast Growth Factor (FGF1) that has the potential to be a first-in-class treatment for multiple corneal conditions.
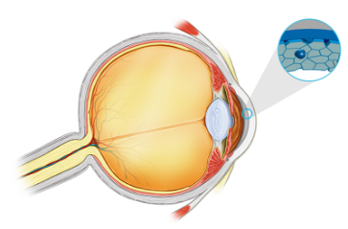
The intracameral injection formulation is being developed for corneal endothelial decompensation associated with ocular surgery and/or Fuchs endothelial corneal dystrophy (FECD).
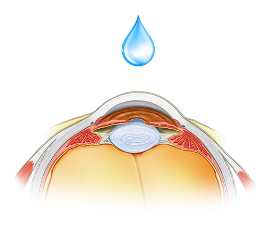
A topical formulation of the product is under development for the treatment of multiple epithelial indications where there is associated ocular surface damage.